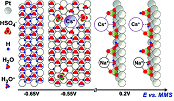
Influence of Cs+ and Na+ on Specific Adsorption of *OH, *O, and *H at Platinum in Acidic Sulfuric Media
The paper authored by
B.B. Berkes,
W. Schuhmann,
G. Inzelt and
A.S. Bondarenko
is published in Journal of Physical Chemistry (2012, vol. 116, pp. 10995–11003).
Abstract:
The influence of Cs+ and Na+ on the adsorption of *H, *OH, and *O (where * denotes adsorbed species) at polycrystalline Pt in acidic sulfuric media has been investigated. Electrochemical impedance spectroscopy (EIS), cyclic voltammetry, and electrochemical nanogravimetry were used (i) to elucidate the models of the interface between polycrystalline Pt and the electrolytes in a wide range of electrode potentials and (ii) to resolve contributions originating from adsorbed *H, (bi)sulfate, *OH, and *O as well as the cations to the overall interface status. Using impedance analysis it was possible to separate at least two adsorption processes: (bi)sulfate and hydrogen adsorption. The nanogravimetry additionally resolves the contribution from Cs+. Specific adsorption of Cs+ at Pt surface significantly affects hydrogen adsorption, while it has almost no effect on the dynamics of SO42- adsorption. Specifically adsorbed alkali cations, however, are desorbed by the onset of *OH(*O) adsorption at the Pt surface. Nevertheless, the cations likely remain in the close proximity to the surface, probably in the second H2O layer, and largely contribute to the formation of the *OH and *O adsorbed species originating from the surface water.
